GP Nord Ltd
Reg. Nr: 40203177406
Address: Kurzemes prospekts 23, Rīga, LV-1067, Latvija
Pārklājs, sterils, ķirurģisks, ar pašlīpošu regulējamu atveri.
50cm x 75cm
75cm x 75cm
Technical Data Sheet
NO.TDS-N72B02-S1-V1.0
 Product Name: Surgical drape with Adhesive Side
Product Name: Surgical drape with Adhesive Side
Classification: Class Is, according to Annex IX, MDD 93/42/EEC
Description: Surgical drape is made of SMMS. There are different design styles: with fenestration or not, with tape or not. It could be used for different operations according to its intended use.
Intended use: Used to isolate the dust and bacteria, prevent the cross infection.
Material: SMMS
Approvals: CE, ISO 13485
Manufacturer: Leboo Healthcare Products Limited
Country of origin: China
Specification: Detail see specification as below.
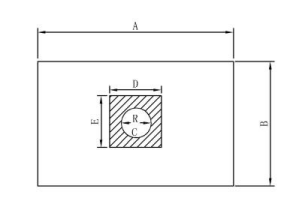
|
Code |
Size |
Measurement Location(cm) |
Weight |
Color |
Style |
||||
|
A |
B |
C |
D |
E |
|||||
|
N72B02-S1 |
50cm x 75cm |
75 |
50 |
7 |
10 |
10 |
45gsm |
Blue |
fenestration φ 7cm, |
|
Tolerance |
±2 |
±2 |
±1 |
±1 |
±1 |
/ |
/ |
/ |
|
Packaging:
(1) Carton Requirements: Five layers corrugated
(2) Packing Quantity: 1pc/pouch, 100pcs/ctn
(3) Carton size: 45*29*38cm
(4) Layers Brown Carton (Paper)
Use limitations (do not use for):
(1) Contact with heavy oils, sparks or flames;
(2) Environment with conditions of excessive heat.
Storage and Disposal:
(1) Store in dry, clean conditions in original packaging.
(2) Store away from direct sunlight, source of high temperature and solvent vapors.
(3) Store with the temperature range -5°C to +35°C and with relative humidity below 80%.
(4) Shelf life is 36 months from the date of manufacture when stored as stated above.
(5) Replace if damaged, heavily contaminated or in accordance with local practice.
(6) Hand and dispose of contaminated products with care and in accordance with national regulations.
Do not wash
Flammable
Do not dry clean
Do not iron Single use
Do not use if package is damaged
| Manufacturer | Leboo Healthcare |
|---|---|
| Product Size | 50 x 75 cm |
| Pieces in package | 1 |